The most advanced batteries in today’s electric cars are based on lithium-ion technology. These batteries use metal-oxide cathodes combined with an encased oxidizer. They are heavy and provide a limited range, up to 160 kilometers (approximately 100 miles) before they need recharging. When compared to internal combustion engine (ICE) technology, with an average range of 800 kilometers (500 miles) between refills, this type of performance appears woefully inadequate and explains why electric cars remain a product for early adopters who see the purchase more as an environmental statement than practical purchase.
What limits lithium-ion battery capacity? If you use lithium-ion batteries for household applications such as powering up electronic devices, these small versions of the battery found in an electric car use a solid oxidizer. The biggest problem for these batteries is heat build up which you probably can attest to if you have ever used a laptop computer for an extended period with it resting in your lap. The batteries that electric cars use replace the sold oxidizer with a liquid one to reduce this exothermic byproduct of the technology.
Better still would be a solution that no longer requires an oxidizer. Instead the battery would breathe in oxygen from the air. And that is precisely what is being developed by a team of researchers at IBM who in January 2012 announced a breakthrough in lithium battery technology. Called the Battery 500 project, the technology is a lithium-air (Li-air) battery capable of storing as much as 1,000 times more energy than current lithium-ion technology.
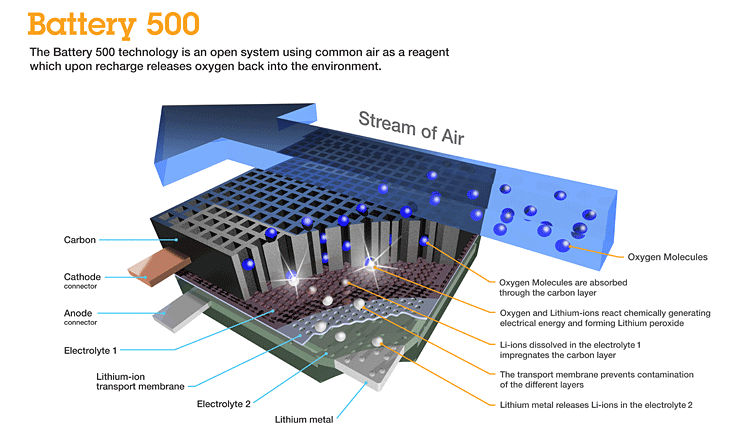
The IBM Almaden Research Lab in San Jose, and IBM Zurich Research Lab in Switzerland have been working on Li-air batteries since 2009. Current models generate 1,500 to 2,000 watts per kilogram. Compare that to lithium-ion technology which generates 150 watts per kilogram, one tenth the energy in a comparable battery size.
Like ICE , Li-air uses oxygen from the air. As the battery discharges to power an electric car the lithium ions reacts with oxygen to form lithium peroxide on a carbon substrate. When recharging the battery the oxygen is released to the atmosphere with the lithium migrating from the carbon substrate back to the battery anode. Because Li-air batteries do not require an enclosed oxidizer within the casing they are much lighter in weight. So not only do you get much higher energy yields, you can adjust the battery size to meet different form factors and mileage requirements.
So why aren’t we using Li-air batteries in cars today? Researchers are trying to extend Li-air battery life. Currently frequent recharges deplete the conducting solution that moves the lithium ions between the electrodes eventually destroying the battery. The breakthrough that IBM announced was the finding of an alternative electrolyte that doesn’t cause the conducting solution to lose its ability to interact with the lithium sustaining a much longer stable chemical reaction. Researchers hope to have a working Li-air battery prototype by 2013 with a commercial product available for electric car manufacturers by 2020.














[…] in April of 2012 I wrote about the evolution of battery technology for electric cars. In the posting I wrote about lithium-ion and lithium-air technology. What I […]