May 9, 2017 – A few weeks ago I attended a seminar at one of Toronto’s preeminent hospitals. The speaker opened with a statement that humanity was on the threshold of a major transformation in the treatment of many medical conditions. He listed wound management, osteoarthritis, musculoskeletal injuries and autoimmune diseases to name but a few. The cure for these conditions, he stated, lay in mesenchymal stem cells.
What are mesenchymal stem cells?
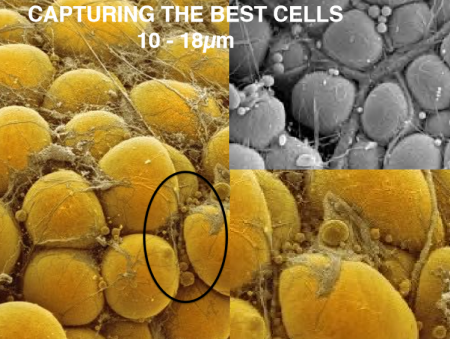
Known by the acronym MSC, these are cells found in our bodies. They are the undifferentiated source of the stromal cells in our bodies, the cells that form all of our connective tissue from organs to the lining of our gut. They can differentiate into bone cells called osteoblasts, cartilage cells called chondrocytes, muscle cells, called myocytes, and fat cells, adipocytes. They are referred to as multipotent because they can differentiate into all these different types of tissue. That’s what makes them so interesting to the field of study known as regenerative medicine.
Scientists have always looked at salamanders and lizards with wonder. How do these creatures drop a tail to a predator as a defense mechanism and then regrow it? Are stem cells involved?
In both cases, the answer is yes. Tail regeneration in both animal species happens when genes send instructions to the stem cells located in proximity to the severed appendage which signals differentiation to begin.
Differentiation is a fascinating aspect of living creatures. An apt analogy is to think of DNA as a movie script with the specific genetic information leading to differentiation, an individual actor’s part. When the actor reads the lines of the script within a scene, those words spoken are akin to the instructions delivered to proteins responsible for transcribing genetic messages to switch on cell machinery that makes an MSC begin to differentiate during cell division into new cell types.
Do humans share the same genes common to lizards and salamanders that can help regrow lost tissue?
Since we share so much of the same DNA, the answer is yes. But in our species, the switch to turn the genes on tends to be restricted or in the off position most of the time. So modern medicine is seeking ways to provide additional stimulus to kickstart regenerative processes. Harvesting our own MSCs appears to be one way to heal ourselves much the way salamanders and lizards do.
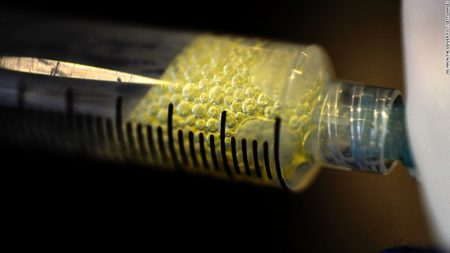
There are two ways to harvest our own MSCs. One is to take a small quantity of the cells and then culture them in a laboratory until you have a significant population. The second is to harvest the cells from our bodies where they can be found in large quantity so that they can be immediately be used in a medical procedure. The MSCs, in either case, are injected into an area of the body where there is damaged tissue and then stimulated to differentiate.
So where do you go in the body to find a significant quantity of MSCs?
If you answered bone marrow, you are wrong. The answer is fat or adipose tissue which has 500 to 1,000 times the concentration of stem cells compared to bone marrow. Adipose-derived regenerative cells are today being used in clinical trials here in North America. Worldwide more than 10,000 patients have undergone regenerative medical treatment using their own MSCs. Here in Canada to date, there have been no clinical trials using MSCs. Canada’s only trials have used embryonic stem cells.
What is wrong with just doing trials with embryonic stem cells?
Embryonic stem cells, as promising as they have been, must be cultured in laboratories to grow a uniform population of sufficient volume for transplantation. What guarantees their genetic stability? The laboratory protocols covering generation and maintenance of cell populations is critical to ensuring that all embryonic stem cells function normally after transplantation without the potential for a mutation occurring in one or more of the cells. A mutation could lead to unintended consequences such as tumors with some potentially malignant.
Embryonic stem cells, when used in treatment, can be derived from the patient but the process to create them involves extracting pluripotent adult stem cells and reverting them to their embryonic state before culturing them to grow a population. Embryonic stem cells can also originate from a third party’s adult stem cells which through reversion can then become useful for regenerative therapies.
MSCs used in regenerative medicine procedures don’t require the complexity that is characteristic in using embryonic stem cells for therapy. They can be harvested from adipose tissue, our body fat, in large volumes, sufficient for therapeutic purposes and can be done with one particular technology (you’ll have to read Part 4 to find out which) in a single minimally invasive treatment process, done under local anesthetic, that involves three steps.
- A patient undergoes liposuction to remove a quantity of abdominal fat.
- The fat is then processed using chemical reagents and lipoaspirate to separate the fat, blood and waste from the MSCs.
- The harvested MSCs are then transplanted into the patient at the affected site requiring regenerative therapy.
So what makes the transplanted MSCs differentiate?
With all cells sharing the same DNA it seems locality determines the type of differentiation that follows. So if you inject MSCs into a knee joint the MSCs will differentiate into chondrocytes. Put them into a liver and they will differentiate into the tissues found in that organ. Put them into a heart, or muscle and voila you will see differentiation produce the desired tissue.
How long does it take before results occur?
Differentiation begins almost immediately as cells replicate. But how does that translate in terms of time before the patient sees improvements? Depending on the medical issue a patient may begin to see improvements within a few days although with some conditions it may take up to six months for the regenerative treatment to work largely dependent on tissue type and normal replication rates through cell division.
In Part 4 of this series on regenerative medicine, I will introduce a technology that may prove to be a game-changer for regenerative medicine. Today, it is being used in a series of clinical trials around the world and its inventors are based in Richmond, British Columbia.














[…] https://www.21stcentech.com/god-cell-story-stem-cells-regenerative-medicine-part-3/ […]