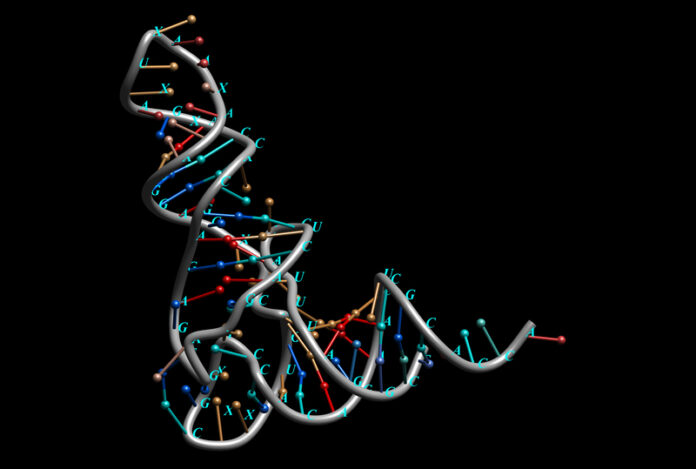
A new vaccine strategy is being developed at the University of California Riverside (UC-Riverside) that uses RNA to build resistance to influenza, COVID and other viruses. The approach would eliminate the need for multiple shots because the vaccine targets a part of the viral genome that is common to all types The work is described in a paper published this week in the U.S. Proceedings of the National Academy of Sciences (PNAS) journal.
One of the paper’s authors, Rong Hai, a virologist at UC-Riverside, notes in an article appearing in a university news feed that the vaccine “is broadly applicable to any number of viruses, broadly effective against any variant of a virus, and safe for a broad spectrum of people. This could be the universal vaccine that we have been looking for.”
How do vaccines typically work? They contain specific information common to a virus and may also include variant strains. When the vaccine is injected, the body’s natural immune system responds to produce T-cells whose purpose is to seek and destroy the biosignatures of the virus wherever found. The body’s immune system also produces B-cells, memory cells that file away the viral information so should an infection occur in the future, it will be armed and ready.
Since the recent global pandemic, we have all become more educated about different types of vaccines and vaccine strategies. COVID-19 with its rapidly evolving strains has required multiple vaccine administrations. The arrival of mRNA-based vaccines thought to be decades away has been revolutionary. Other techniques employing adenoviruses and attenuated live viruses have undergone enhancement to help fight viruses. The common theme, however, in all of these vaccines is they are individualized solutions that target specific viruses only.
The UC-Riverside vaccine strategy is different. Scientists have been identifying common characteristics in all viruses that can be a marker for the body to kill any of them and create memory immunity to stop viruses in general in the future. If successful, this research will be a medical game-changer.
Currently, UC-Riverside is testing its vaccine on mice in laboratory settings. The vaccine is using a live but modified version of a virus containing silencing RNA molecules. Silencing RNA molecules are non-coding sequences of RNA that help to regulate gene expression. They are molecular “off switches,” that prevent specific genes from being expressed.
When injected into mice with different viral infections, in this case, a virus called Nodamura, their response has been to produce RNA Interference proteins or RNAi that weaken the virus eventually knocking it out.
In animals like mice and humans, RNAi’s purpose is to deactivate, genes. Viruses produce proteins to block RNAi. The vaccine, however, uses a modified virus that weakens its protein-blocking capability. Incorporated into the vaccine, it becomes a useful component for boosting natural immunity.
The vaccine in laboratory settings using mice that lacked both T and B-cells has protected them from lethal doses of Nodamura for 90 days which in mice is equivalent to 900 days of protection in humans. Even newborn mice have been protected by the vaccine making it likely that this could be used with babies under six months of age.
UC-Riverside has been awarded a U.S. patent for its RNAi technology. Going forward the university plans to produce RNAi vaccines for influenza that can be administered using a nasal spray.
The researchers believe that targeting the viral genome using RNAi means no virus can escape them. All they need to do is use “cut-and-paste” to create one-and-done vaccines for Dengue, SARS, COVID and other viruses.
RNAi and mRNA therapies are rapidly changing how humans combat disease. We already know about mRNA vaccines used to save tens of millions of lives during the COVID-19 pandemic. Now we can add RNAi treatments. Recently, Patisiran has received U.S. FDA approval for treating polyneuropathy in people suffering from hereditary transthyretin-mediated amyloidosis, a disease that affects 50,000 people globally and often leads to death. Patisiran is the first RNAi-based drug that has received approval.
It will be interesting to see how quickly RNAi vaccines move to human trials and then FDA certification. If successful, they will be powerful tools in our hands for combating the viruses we know and the ones that may leap to us in the future, in particular, avian influenza which recently has made the leap from birds to cows.