June 5, 2014 – Back in April of 2012 I wrote about the evolution of battery technology for electric cars. In the posting I wrote about lithium-ion and lithium-air technology. What I didn’t know was that a company named Phinergy was working on a different type of metal-air battery using aluminum and zinc plus air.
This week Phinergy, an Israeli company, along with the aluminum giant, Alcoa Canada, demonstrated an electric vehicle (EV) capable of driving 1,800 kilometers (over 1,100 miles between charges) using a combination of aluminum-air and lithium-ion storage technologies. The Phinergy aluminum-air battery at 100 kilograms (220 pounds) weight contained enough on board energy to allow the vehicle to travel up to 3,000 kilometers (over 1,860 miles). Compare that to the best, current lithium-ion batteries in the Tesla Model S sedan. At best they can do less than 500 kilometers (310 miles) on a single charge and the on board battery weighs 5 times as much.
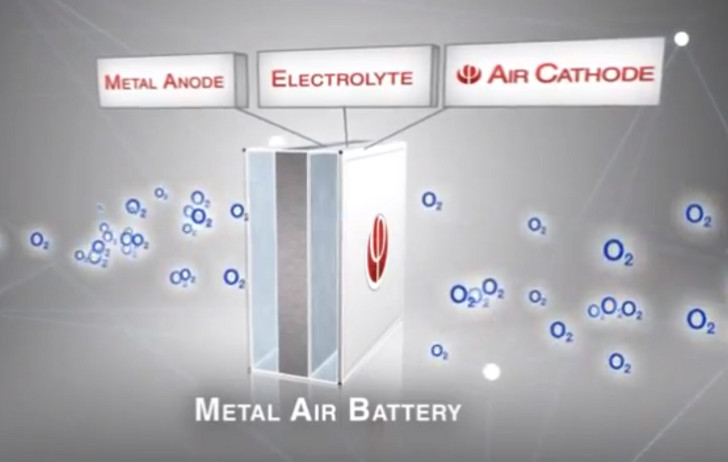
How does an aluminum-air battery work? They use an air-electrode capable of breathing ambient air and extracting the oxygen from it. Compare this to traditional batteries which store and release oxygen from chemicals contained in a liquied or solid cathode. An air battery doesn’t need to replace or recharge its cathode. And an air battery is far lighter. The combination means significantly more power for a longer period of time.
Phinergy batteries use a porous electrode with a large surface area that captures the oxygen from ambient air. The electrode also contains a silver-based catalyst that doesn’t let CO2 interact with it. This unique and proprietary catalyst solves a common problem in air-battery technology, carbonization caused by CO2 permeating the electrode.
To make the aluminum-air battery even more economical they are produced in areas where electrical energy capacity and cost is low. In the case of the demonstration EV this week, the battery was forged at the Alcoa smelter in Baie-Comeau, Quebec where the company can draw on a significant hydroelectric power resource.
Aluminum-air batteries do break down over time. As they drain the metal turns into aluminum hydroxide. When spent the entire battery can be recycled to forge new aluminum-air batteries. For the air-battery operator it will mean swapping out the old battery for a new one every few months. This could be done at service centres which would keep an inventory of these batteries in supply. Tesla demonstrated its plans for charging and swap out service centres back in July of last year. In the Tesla demo the battery was swapped out in 90 seconds. So this notion of a quick battery replacement service that is as fast as refilling a conventional gas or diesel tank seems very doable.
In the case of the test car demonstrated at the Canadian International Aluminum Conference on Wednesday in Montreal, it was outfitted with both an aluminum-air and lithium-ion battery system. The notion behind this was that the EV could run on its lithium-ion charge when driving on short urban trips of 50 kilometers (31 miles) or less, but when used for longer trips the aluminum-air battery would kick in. To feed the chemical reaction from the aluminum-air battery drivers using a test car like this would add tap water every month or two to feed the chemical reaction.
Phinergy is also experimenting with other metal-air technologies. They have developed a zinc-air battery that has some advantages over aluminum. Zinc-air is extremely durable. A battery can last thousands of hours without chemical deterioration.
The company hopes to see metal-air batteries made with aluminum and zinc become the primary storage devices for transportation, for backing up renewable power sites, for electronic devices and for industry and defense.

















I’m going to use approximate numbers, give or take 10%, in this analysis of the aluminum battery proposition. LME prices on bulk aluminum, which is about as cheap as aluminum by the ton will get, average about $1.00 per pound (unless increases in demand for aluminum batteries boosts prices). According to Phinergy claims, their battery energy density seems an excellent, but doubtful, 3.6 kWh/pound. Hence best possible ideal economic case must be $0.28 worth of aluminum consumed per kWh produced. But the real world “best case” is far from the actual case. Factoring in transportation, fabrication, silver catalyst, recycling, labor, management, and capital costs, the actual total costs would likely exceed $0.75 per kWh delivered to end-users by aluminum batteries.
Gasoline energy density is 6 kWh/lb. In the Chevy Volt, the ICE only converts about 30% of that, say 2 kWh, into propulsion power (that’s a little tricky to calculate because under most conditions when the ICE runs, the Volt engine is mechanically coupled to the wheels, bypassing the electrical drive motor). So, the first glance economics of ideal aluminum batteries look similar to, but not significantly better than, the Chevy Volt. But if you add another 300 lbs of Lithium batteries to the Volt, its battery-only range increases to ~ 60-miles. In that case the Volt wins hands down. Using aluminum metal as ground transportation fuel seems silly. Why not use the aluminum battery to power commercial and military aircraft, especially drones?
Hi Al, I’m still trying to understand the reference to the Volt “winning hands down” in a comparison.
GM claims the initial Volt battery can store and discharge cycle enough energy to drive the Volt over 100,000 miles. The Phinergy aluminium/air battery must be physically replaced every 1,200 miles. It’s not rechargable. The Phinergy people don’t tell us what their aluminum battery costs, but it’s a very safe bet it will cost a lot more than 1/83 of the Volt battery costs. For all we know, the Phinengy battery could actually cost more than a Volt battery. Doubling the Volt’s battery range to 60-miles means that in most cases its ICE only runs 1/10 as much as the engine in the present 30-mile-range Volt’s. Then the market for Volts would expand exponentially.
But no, instead of rationally extending the Volt’s range with a larger battery, GM seems to have gone narcissistically nuts by introducing a high styled ELR Cadillac coupe version of the old Volt driveline that is priced similar to a Tesla S. Get serious. It’s just a tarted up Volt. Does GM have a death wish? Has the oil cartel placed undercover sabotage agents in GM’s top management? Someone at GM made the decision that the Chevy Volt ICE would require premium fuel. How dumb is that? What GM genius figured luxury car buyers would lay Tesla S dollars down to purchase a tarty Volt just because it has a Cadillac emblem and fake pearl necklace on it? The driver who buys a Tesla S gets a plush high-styled all electric silent road rocket that handles great and runs a couple hundred miles on pure battery power. The John who buys a ELR gets expensive flash, but under the slathered on trashy makeup it’s still just a Volt.
@Al,
One problem with you analysis, even if correct for the initial battery, is it assumes the recylce cost would be the same as the initial manufacturing cost. If that cost is only 99% energy from a hydroelectric power resource and water, it could still make ecconomic sense despite a high upfront cost to produce an initial battery. Definitely not an order of magnitude better than other technolgies, so not likely to be more than niche market any time soon.
[…] via Aluminum-air battery demonstrates extended range for EVs.. […]
[…] by sidcool1234 [link] [1 […]
[…] https://www.21stcentech.com/aluminum-air-battery-powered-car-travels-1800-kilometers-recharge/ […]
[…] Continue Reading : New Aluminum-Air Battery Powered Car Travels 1,800 Kilometers Without a Recharge […]
[…] Coches híbridos, vehículos eléctricos… la ciencia está innovando continuamente en este campo. El más reciente descubrimiento viene de la mano de una compañía llamada Phinergy, y es que han logrado crear una batería de […]
[…] eléctricos… la ciencia está innovando continuamente en este campo. El más reciente descubrimiento viene de la mano de una compañía llamada Phinergy, y es que han logrado crear una […]
[…] Coches híbridos, vehículos eléctricos… la ciencia está innovando continuamente en este campo. El más reciente descubrimiento viene de la mano de una compañía llamada Phinergy, y es que han logrado crear una batería de […]
The only really new ideas here are using aluminium instead of zinc, and the admittedly clever silver catalyst. The catalyst prevents conversion of hydroxide into carbonate or bicarbonate (carbonation), which would be caused by carbon dioxide in the air reacting with the hydroxide in the battery electrolyte. However, carbonation in metal air batteries is only a significant problem in rechargeable batteries, which this is not. So unless they intend to develop a rechargeable version, it is not clear why preventing carbonation is necessary – and hence the catalyst should not be required.
Zinc air batteries, which work on the same principle, have been around since the 1930s. Using aluminium may seem like a clever idea, because aluminium has a higher redox potential than zinc, and is also lighter than zinc. So it enables a higher energy battery with less weight. The high range enabled by the aluminium air battery is a huge advantage, as is the quick battery replacement time. However, the fact remains that it is still a primary, non-rechargeable battery, with all the same problems as existing primary batteries.
The biggest of these problems is recycling / re-use. The energy provided by primary batteries doesn’t come for free. It comes from the chemical energy stored in the battery – in this case, from the highly reactive aluminium metal. When the battery is spent, the aluminium metal has been converted to aluminium hydroxide, and converting that back to aluminium metal again takes a lot of energy – a lot more energy than the battery supplied in the first place. The same is true for zinc air batteries, because those convert zinc metal into zinc hydroxide, which must also be converted back into zinc metal in order to be re-used in a battery.
Converting aluminium hydroxide into aluminium metal is a very energy intensive process. First, the aluminium hydroxide has to be heated to convert it into aluminium oxide. Then, the aluminium oxide has to be transferred to an aluminium refinery, where it is dissolved in molten cryolite and electrolyzed. This requires enormous amounts of electricity – an aluminium electrolysis factory typically consumes the output of an entire power station.
Even if all the electricity required came from renewable sources, it still isn’t a carbon-neutral process. This is because the aluminium electrolysis cells use carbon electrodes, and because they require heating to keep the aluminium oxide / cryolite mixture molten. The heating could be done electrically, but that would require even more electricity, so it is usually done with hydrocarbon fuels such as natural gas. Also, since the electrolysis happens at high temperatures, the oxygen produced at the carbon anode immediately reacts with said anode and is converted to carbon dioxide. This reaction alone means that 1.2 tons of carbon dioxide is directly produced for each ton of aluminium metal produced.
If you add in the heating fuel used and the transportation fuel required for transporting all the spent batteries to the aluminium factory, then the actual amount of carbon dioxide emitted per ton of aluminium produced is likely to be at least twice as much as the amount directly produced in the electrolysis cells. All of which means that the large scale use of aluminium air batteries is unlikely to be economical, and will never be carbon neutral unless entirely new technologies for refining aluminium are also developed.
[…] Coches híbridos, vehículos eléctricos… la ciencia está innovando continuamente en este campo. El más reciente descubrimiento viene de la mano de una compañía llamada Phinergy, y es que han logrado crear una batería de […]
[…] The two companies have demonstrated an electric vehicle that is capable of driving 1800 kilometers with the deployment of a technology that combines lithium-ion storage and aluminium-air. […]
[…] Fuente: 21stcentech […]
[…] Customer RatingsWill Nanotechnology be the Answer for the Next Generation of Lithium-Ion BatteriesAluminum-air battery demonstrates extended range for EVs.Silicone Sponge BatteriesUniveristy of Alberta Researchers Develop Next Generation BatteryBatteries […]
[…] company Phinergy recently revealed that one of their creations powered an electric car for approx 1800km on a single charge using air….that’s right AIR!!! And because I did not complete my Doctorate in Enginering I will […]
[…] New Aluminum-Air Battery Powered Car Travels 1,800 Kilometers Without a Recharge […]
[…] promising area is aluminium-air batteries, which have been placed in a car to deliver a whopping 1,100 miles on a single charge. Then there […]
[…] promising area is aluminium-air batteries, which have been placed in a car to deliver a whopping 1,100 miles on a single charge. Then there […]
[…] promising area is aluminium-air batteries, which have been placed in a car to deliver a whopping 1,100 miles on a single charge. Then there […]
[…] promising area is aluminium-air batteries, which have been placed in a car to deliver a whopping 1,100 miles on a single charge. Then there […]
[…] promising area is aluminium-air batteries, which have been placed in a car to deliver a whopping 1,100 miles on a single charge. Then there […]
[…] promising area is aluminium-air batteries, which have been placed in a car to deliver a whopping 1,100 miles on a single charge. Then there […]
[…] promising area is aluminium-air batteries, which have been placed in a car to deliver a whopping 1,100 miles on a single charge. Then there […]
[…] promising area is aluminium-air batteries, which have been placed in a car to deliver a whopping 1,100 miles on a single charge. Then there […]
[…] promising area is aluminium-air batteries, which have been placed in a car to deliver a whopping 1,100 miles on a single charge. Then there […]
[…] promising area is aluminium-air batteries, which have been placed in a car to deliver a whopping 1,100 miles on a single charge. Then there […]
[…] promising area is aluminium-air batteries, which have been placed in a car to deliver a whopping 1,100 miles on a single charge. Then there […]
[…] promising area is aluminium-air batteries, which have been placed in a car to deliver a whopping 1,100 miles on a single charge. Then there […]
[…] promising area is aluminium-air batteries, which have been placed in a car to deliver a whopping 1,100 miles on a single charge. Then there […]
[…] promising area is aluminium-air batteries, which have been placed in a car to deliver a whopping 1,100 miles on a single charge. Then there […]
[…] promising area is aluminium-air batteries, which have been placed in a car to deliver a whopping 1,100 miles on a single charge. Then there […]
[…] promising area is aluminium-air batteries, which have been placed in a car to deliver a whopping 1,100 miles on a single charge. Then there […]