August 7, 2017 – When my daughter was born back in 1984 she came into the world with a faulty heart. She had what is defined as a congenital (as in born with) disease that could not be repaired until she grew to a safe enough size to survive surgery. My wife and I were given the task to grow her, whatever it took, so that she could undergo a nine-hour procedure to repair as best as possible what nature had dealt her. The condition was Tetralogy of Fallot with Absent Pulmonary Valve, a complex heart, and lung congenital disease. My wife and I became experts in this particular heart ailment. We read the latest scientific papers, conducted interviews with pediatric cardiologists and lung specialists, and advocated for a multi-disciplinary, holistic treatment program to save our daughter’s life. We were helped by a visit to the Mayo Clinic in Rochester, Minnesota, where we tapped into the brain trust there on the latest understanding of the physiology and genetic origins of the disease.
We were lucky to some degree. Our daughter survived multiple bouts of pneumonia, two open-heart surgeries, and a pulmonary valve implant done by cardiac catheterization. Today she is 33 and married. Her prognosis is good but that doesn’t mean she won’t need further surgical interventions. And it doesn’t mean that she is not susceptible to respiratory infections and diseases that can compromise both heart and lung function.
She is an artifact of a time and place when the science and technology to combat congenital heart disease was still about cut and cover. But this is changing.
Enter Gene Editing Tools
The future for children like my daughter may soon be far different because of a tool called CRISPR/Cas9, a gene editing technology that I have talked about in the past at this blog site. CRISPR is the most well known of a number of gene editors capable of altering our DNA, as well as that of every animal and plant on the planet. It is helping to create disease and pest resistant crops. It is eliminating disease carrying mosquitoes. And now in humans, it holds the promise of removing unhealthy genetic information and replacing it with healthy DNA sequences. The ultimate capability of this technology is the elimination of all congenital diseases by snipping out the bad and replacing it with the good.
In the latest headlines reported in the last couple of weeks we have learned of an experiment conducted in the United States that targeted malfunctioning genetic information responsible for a congenital heart condition known as hypertrophic cardiomyopathy. This disease is very different from what my daughter had. But it has something in common. The disease is related to gene mutations that cause cells in the body to become disrupted leading to abnormal growth. My father-in-law succumbed to this disease in his 97th year. He was one of the rare ones who didn’t show signs of the condition until he was in his mid-80s. At that time he was outfitted with a cardiac pacemaker to help alleviate the worst symptoms of the condition which causes the wall between the two lower chambers of the heart to thicken to a point where it no longer is supple enough to contract. Hypertrophic cardiomyopathy normally begins to show symptoms in the population of adolescents and young adults. When it does it tends to lead to sudden cardiac death.
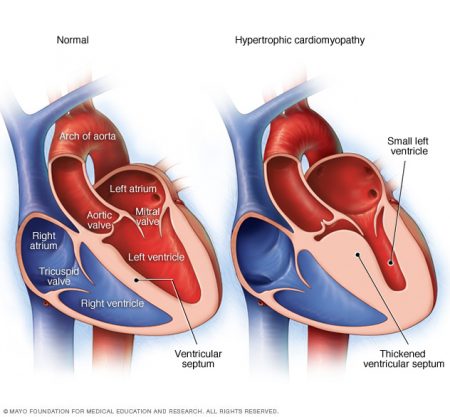
Like my daughter’s congenital heart condition, hyptertrophic cardiomyopathy (seen in the image above on the right) is associated with a range of gene mutations on one or more chromosomes. The research to date indicates 8 different gene mutations are linked to the disease. One of those genes is the MYBPC3 mutation which can be passed on to the child by either parent. It is defined as dominant inheritable trait which means only one copy is needed from a parent for the child to exhibit the mutated gene.
What does MYBPC3 do?
It provides important instructions that affect the contractions of the heart muscle. Specifically, it alters sarcomeres, the cells which make up the thin and thick filaments within the heart muscle (see image below). When the mutation is not present the sarcomeres work conjointly to create normal contractions. But when the MYBPC3 mutation is present the protein carrying instructions responsible for creating the heart produce errors in the natural configuration of these cells. And although the mutation may be asymptomatic, in 30% of cases the heart muscle will thicken to a point where symptoms occur.
The latest research from Oregon Health and Science University, Portland, that was published recently in the journal, Nature, attempted an experiment where mutated MYBPC3 genes were targeted using CRISPR/Cas9 to substitute good genetic information for bad. A total of 58 human embryos not destined for implantation were targeted. The team successfully corrected the genetic information responsible for the defect in 42 of the embryos while not causing any unwanted genetic changes in the remainder of the genome. Normal copies of the MYBPC3 gene were implanted using the Cas9 protein which serves as the knife tool within CRISPR. First Cas9, working with the embryo’s own RNA guide molecules selected the place for editing the gene. Once the defective gene was excised, it was replaced with healthy genetic information. Upon completion, the cell’s machinery carried on as if nothing had happened.
Such a technology, if replicated in treating other congenital diseases at the embryonic stage, would be revolutionary. Then parents who were known carriers of harmful genetic mutations based on DNA analysis and a comprehensive family history could have doctors intervene at the embryonic stage to correct life-harming and life-ending defects.
Of course, the dark side of speculation by journalists covering the story led to headlines that declared “designer babies” are in our future. Parents with perfectly normal medical histories and DNA exhibiting little in the way of harmful mutations could decide they wanted a child with blue eyes even though both of them had brown. Or some could request doctors to alter other genetic information that could bestow on an unborn child improved physical strength or mental acuity.

















