April 13, 2018 – I never thought about where gene drives and genomics are taking biomedicine until today when I read that one of the largest biopharma companies, GlaxoSmithKline announced it was selling off its gene therapy research and products. As one business analyst stated, it is pretty obvious that curing patients with intractable diseases may be bad for the bottom line.
The pace of current biomedical progress is being driven by a number of new technologies including:
- Stem cell therapies
- Gene editors
- 3D-Bioprinting
In the case of Glaxo, it was their research based on the use of gene editors that was raising alarms. Gene editors like CRISPR/Cas9 are making it possible to cure rare diseases in what is turning out to be one-time therapeutic treatments. For a company like Glaxo, one-and-done does not do much for the bottom line unless you charge millions of dollars each time you employ the cure. But one-and-done gene therapy is becoming the future of medicine particularly in dealing with diseases of the immune system, and diseases linked to defective genes. With CRISPR/Cas9 or other DNA slice and replace tools we may soon see an end to sickle cell anemia, muscular dystrophy, cystic fibrosis, hypertrophic cardiomyopathy, Huntington’s, Amyotrophic Lateral Sclerosis (ALS), and various cancers, all formerly incurable diseases.
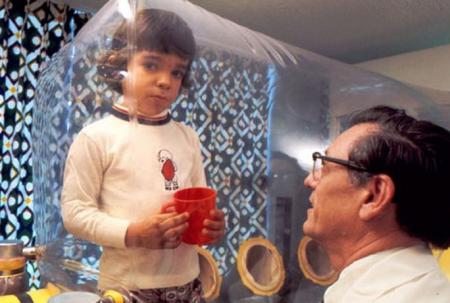
One of the therapies Glaxo was selling is Strimvelis, which when it was announced in 2016, was described as a major breakthrough in treating extreme immunodeficiency. The disease, popularly known as “bubble-boy syndrome” (see picture below), goes by a more formal name, Adenosine Deaminase Deficiency. Strimvelis inserts a healthy adenosine deaminase gene into patient stem cells extracted from bone marrow. The agent carrier Glaxo uses is a retrovirus. The end result is stem cells that start replicating with the healthy gene on board, and which can then be reinserted into the patient to effect a cure.
Why is this so expensive? Because each treatment is a one-off. So it isn’t like an aspirin or acetaminophen which get produced en masse. And unlike the latter two, there is no repeat business. One dose equals one cure. And since globally there are only 350 cases of “bubble-boy syndrome,” where’s the profit?
Glaxo isn’t the only bio-pharma company looking at the bottom line impact that its research into gene editing will have on its profits in the coming years. That may explain why in other areas of drug development companies like Glaxo are developing biologics, a new category of drugs, many of them requiring infusion or injections, that are aimed at providing relief to patients suffering from more common and chronic ailments. But with biologics and the method by which the drugs are delivered, the biopharma companies can justify much higher prices than the cost of an aspirin.
One thing is for sure. For big biopharma, there appears to be no long-term profit inducement to using new technologies like gene editors to cure rare diseases. That’s why, I believe, it may make more sense to take this type of research out of their hands and place it into government laboratories, medical not-for-profit foundations, research-centered hospitals, and universities.