Hydrogen is the most abundant element in the Universe. It comprises 75% of all matter. But here on Earth, it isn’t found in isolation but rather in compounds, the largest being water (H2O). Hydrogen powers the Sun so we know its energy potential is high. If we were to extract the hydrogen from a litre of water, we would get twice as much hydrogen compared to the water.
That’s why researchers have been trying to develop technologies to harvest hydrogen from water, a zero-emission alternative to current technologies that extract the gas from fossil fuel natural gas.
When you look around this planet, where is there lots of water? The answer is obvious, the oceans that cover more than 70% of the planet. So one would think, to harvest hydrogen from this vast resource would be a no-brainer. But there are problems in developing practical, durable, hydrogen extraction technologies from seawater.
Using electrolysis, where electricity is applied to electrodes placed in water, is the current method for separating hydrogen and oxygen molecules. There is an entire industry building electrolyzers which produce hydrogen this way. The water has to be fresh and purified because any mineral contamination can corrode the electrodes and shorten the life of the equipment. Is it economic to harvest hydrogen this way? If the electricity being used by the electrolyzers comes from air-polluting sources, then the hydrogen produced cannot be described as green. And if the energy input costs are greater than what can be derived from the hydrogen when burned, then there is little point in producing it.
Then there is the saltwater problem. Saltwater contains lots of minerals and mostly salt. When electrolysis happens in saltwater, it quickly corrodes the electrodes. That means conventional electrolyzers will not work for long when using saltwater as the hydrogen source.
There is a two-step process to harvest hydrogen from seawater, The first step involves desalinating the water. Then conventional electrolyzers can be used to harvest the hydrogen. Why this is not being done commercially is because the extra step makes the process cost prohibitive. That’s why research teams from universities around the world have been looking for technology breakthroughs. They have come in fits and starts.
In 2010, a team at the University of California, Berkeley, tried by inventing an electrocatalyst made from a molybdenum-oxo complex which when combined with a mercury electrode produced hydrogen. Nothing commercially applicable came out of this invention.
In 2019, a Stanford team made an electrode from nickel-iron hydroxide and nickel sulphide that harvested hydrogen from seawater. It worked for 1,000 hours before the electrodes corroded.
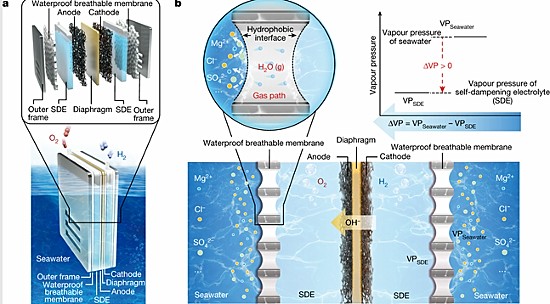
A different approach from a team of researchers from Nanjing Tech University in China just recently announced appears to be quite promising. They have created a membrane-based seawater electrolyzer to harvest hydrogen and published their findings in November 2022 in Nature.
In the paper, the team describes the challenges that have plagued all prior attempts to harvest hydrogen from seawater and described their strategy to overcome the corrosion issue. Instead of putting the electrodes into seawater, they invented a concentrated potassium hydroxide electrolyte solution in which to place them. They also created a fluorine-rich porous membrane to separate the seawater from the electrolyte. The membrane blocked the seawater from interacting with the electrodes and only allowed water vapour to penetrate through it. The beginning of this article illustrates how the exchange is produced.
With this new technique, the Nanjing team has been able to demonstrate stable hydrogen harvesting without corrosion. Its tests using a current density of 250 milliamperes per square centimetre with electricity coming from renewable sources ran for over 3,200 hours continuously producing no corrosion of the electrodes.
The inventors believe this technology can be adapted on an industrial scale which would mean electrolyzers for processing seawater. It also believes operational costs should be similar to current freshwater-using electrolyzers. Another advantage of their invention is it can be used with other water-based sources since only water vapour interacts with the electrodes. That means even wastewater and other contaminated sources of effluent can be used for hydrogen harvesting.
What’s the upside to developing a hydrogen from seawater energy model? The potential of capturing ocean hydrogen can give the planet an almost unlimited source of 100% zero-emission energy for powering manufacturing processes, fueling transportation and heating and cooling homes and buildings. It is another step in moving us away from our current fossil fuel energy dependency.