June 25, 2018 – Carbon sequestration has been talked about for many years even before the climate change debate went global. The phenomenon is responsible for keeping the total carbon volume of the planet in different states. Trees sequester carbon. The ocean, lakes, and rivers sequester carbon. Peat bogs and marshes sequester carbon. Permafrost sequesters carbon. Even our bodies sequester carbon, that is until we die. We call these natural forms of carbon sequestration, carbon sinks. But most of us don’t associate rocks with carbon sequestration. And we’re wrong.
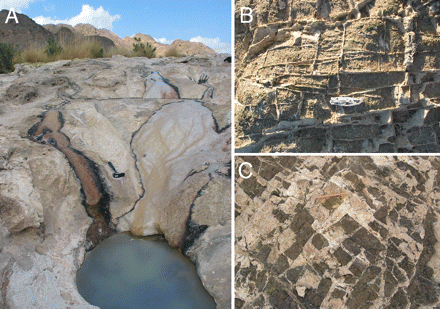
Natural formations of carbonate rocks called peridotite can be found wherever there are igneous rocks formed from magma reaching the Earth’s surface. Peridotite is quite common for a rock type that most of us have never heard of. Where you find peridotite surface deposits, from igneous upwellings you have the means to sequester carbon by creating solid carbonate minerals.
Peridotites are easily weathered when exposed to water or low temperatures and it is this characteristic which has made the rock an interesting candidate to become a significant carbon sink. In a 2008 published paper in the journal PNAS, Proceedings of the National Academy of Sciences of the United States of America, scientists Peter Kelemen and Jürg Matter of the Lamont-Doherty Earth Observatory, Columbia University, describe the processes of natural carbonation in peridotite and how it can be enhanced to become a significant sink for atmospheric carbon dioxide (CO2). They then go on to discuss ways to enhance the rock’s carbon sequestration properties through drilling, hydraulic fracturing and pumping liquefied CO2 into mineral formations. An additional step, heating the CO2 could increase in situ carbonation. Kelemen and Matter’s work in Oman where large exposed peridotite deposits can be found. They estimated that with little expenditure of energy these Oman rocks could store in excess of 1 billion tons of CO2 annually. That is equivalent to about 2.5% of the current annual output of CO2 produced by human activity on the planet today.
If we found 40 similar world sites similar to the one in Oman we could remove the CO2 annually produced by human activity, permanently sequestering it in rock formations. It should be noted that we would still be left with current CO2 levels of greater than 410 parts per million. But by adding additional peridotite sequestering sites we could bring that number down to levels considerably lower, ensuring the planet’s atmospheric temperature would not continue to rise.
What is the process by which rocks sequester carbon?
It is called mineral carbonation, a process where atmospheric CO2 in the presence of water binds with different types of rocks to form stable carbonate minerals, such as calcium carbonate (calcite and limestone), and magnesium carbonate (magnesite).
Is this a better carbon sequestration solution than current CCS technologies?
Undoubtedly because all we would be doing is enhancing a natural process and fixing the carbon into a stable mineral. That sure beats capturing CO2 from the smokestack of a coal-burning power plant and then pumping the gas in high-pressure liquefied form into oil wells to enhance petroleum recovery such as is being done at Boundary Dam in Saskatchewan.
Peridotite carbon sequestration would involve applying an incidental amount of technology to a natural process to speed it up. No more, no less.
In the September 2013 issue of the Journal of Petroleum Science and Engineering, the costs and methods for mineral carbonation are laid out in an article entitled “A review of mineral carbonation technology in sequestration of CO2.”
What blows me away about the discovery of peridotite’s natural carbon sequestering capabilities are the dates associated with the research. I’m citing journal articles from 2008 and 2013. But the New York Times on the weekend published an article about it as if it were new science. Considering the billions of dollars that have gone into CCS projects globally, wouldn’t mineral carbonation of naturally occurring peridotite found on the Earth’s surface have been far less costly? And wouldn’t we by now have created pilot projects and fully operating sites for mineral carbonation for a fraction of the cost we are currently spending on CCS and other carbon emission reduction strategies?