Yesterday, I wrote about the invention of solvents that capture carbon dioxide (CO2) from the air streams of chimneys at industrial sites. The novel technology could make carbon capture utilization and sequestration (CCUS) a much more affordable option than the current methods in place and planned.
Today, I’m writing about removing CO2 from the ocean. Why do we want to do this? A significant amount of anthropogenic-derived CO2 gets absorbed into ocean water. The ocean is the world’s largest carbon sink (30 to 40% of all the CO2 humans produce) and since the start of the Industrial Revolution, it is adding more CO2 than in previous times.
We measure the alkalinity or acidity of water using the Power of Hydrogen or pH scale which measures whether hydrogen ions are increasing or decreasing in response to the amount of CO2 being absorbed by ocean water. Added CO2 in ocean water creates a weak carbonic acid (H2CO3) that causes chemical changes that increase hydrogen and bicarbonate ions and decrease carbonate ions. The latter helps crustaceans along with calcium to form shells and exoskeletons.
The pH scale is logarithmic and runs from 0 to 14. Neutral is 7. Anything above is defined as basic or alkaline. Anything below is acidic. Ocean water has an average pH of 8.1 which is down 0.1 (on a logarithmic scale that’s a big deal) since we first started using this measuring system.
The ocean isn’t the only CO2 absorber. Freshwater lakes where pH levels are close to neutral also act as carbon sinks. Acid rain back in the latter part of the 20th century contributed to the acidification of freshwater lakes and rivers. Governments mandated smokestack scrubbers to remove the chemicals that were responsible for this environmental damage. Today, in North America, acid rain is no longer an environmental hazard.
Whether ocean water or freshwater, increasing acidity disrupts life at the bottom of the marine food chain. The increase in CO2 absorption by the world’s oceans has increased acidification by 30% in less than two centuries. The damage can be seen in coral reefs, shellfish and other marine life.
Nature has its own solutions for dealing with more CO2 entering the ocean or fresh water. Algae, seagrass, and kelp absorb the extra CO2. But since the Industrial Revolution and particularly in the last few decades, natural systems for absorbing CO2 in the ocean are having trouble keeping up with the volume of gas entering the marine environment.
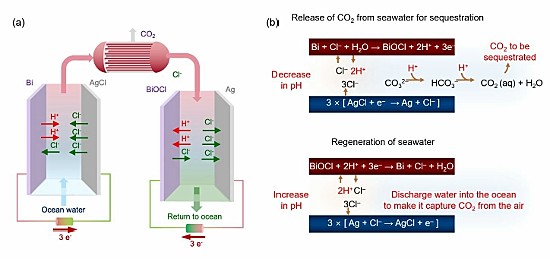
That’s where a team of researchers at the Massachusetts Institute of Technology (MIT) may have come up with a technological fix. In a recently published paper appearing in a February 2023 edition of the journal Energy and Environmental Science, these researchers describe what they call an “asymmetric electrochemical system” that can remove CO2 continuously from ocean water.
The authors note in their paper that there has been a discrepancy between the development of emission reduction technologies for the atmosphere versus those for the ocean. The former has been extensively discussed in past articles on this blog site and includes the invention described yesterday. With the exception of the latter, CO2 removal from the air technologies has proven so far to be prohibitively expensive.
Not so, state the creators of this MIT invention which uses electrochemical modulation to initially separate the CO2 from ocean water. It then alkalizes the treated water before returning it to the ocean. It does not need special and expensive membranes or additional chemicals. It is easy to deploy, forms no harmful byproducts, and in initial testing on simulated seawater, has been both energy efficient and inexpensive.
So what do they propose to do with the removed CO2? The authors write it “can be injected directly…into sub-surface geologic structures for long term sequestration, or used as a feedstock for fuels or for commodity and specialty chemicals production.” They also note that the technology can be installed on floating platforms, colocated with floating wind farms and marine solar installations, on cargo ships, or integrated with desalination plants already handling volumes of ocean water.