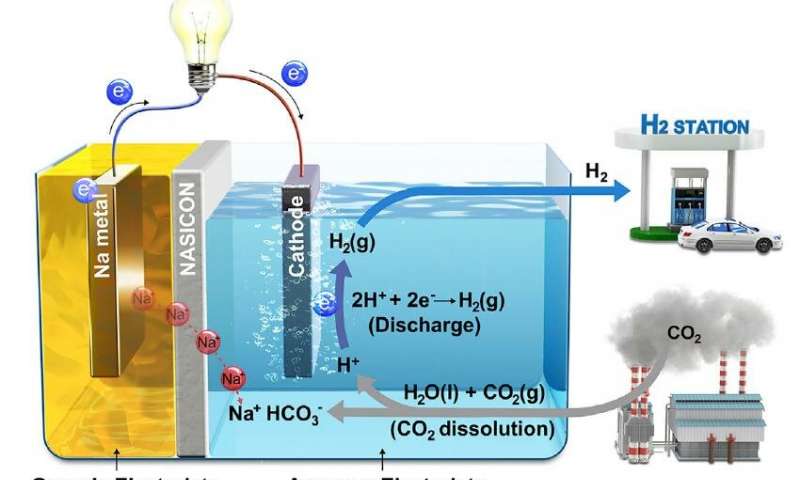
February 5, 2019 – South Korean Ulsan National Institute of Science and Technology (UNIST) and Georgia Institute of Technology researchers have published their work on a breakthrough system designed to produce electricity and hydrogen (H2) while eliminating carbon dioxide (CO2). They have created a hybrid Sodium (Na)-CO2 cell that resembles a fuel cell and continuously produces electricity while eliminating the greenhouse gas. The byproduct, H2 can be used as a clean energy source as well.
Guntae Kim, of the School of Energy and Chemical Engineering at UNIST states, “Carbon capture, utilization, and sequestration technologies…recently received a great deal of attention for providing a pathway in dealing with global climate change. The key to that technology is the easy conversion of chemically stable CO2 molecules to other materials…Our new system has solved this problem with CO2 dissolution mechanism.”
The Na-CO2 cell contains a cathode made of metallic sodium, a sodium superionic conductor, or NASICON, and an anode immersed in water. A lead wire connects cathode to anode. When CO2 is injected into the water the electrochemical reaction eliminates the greenhouse gas at a conversion rate of 50%. In testing, as of the January 21, 2019 publication date of their research, the cell has remained stable more than 1,000 hours without showing deterioration in any of its components.
What is the significance of this invention?
Carbon capture, utilization, and sequestration (CCUS) is a preferred technology for eliminating CO2 from the atmosphere. Unlike carbon capture, and sequestration (CCS), there is no need to find a stable storage medium for the CO2.
If a Na-CO2 cell were to be sufficiently large and attached to existing coal-fired power plants it could conceivably eliminate all of the CO2 emissions from the source while improving the energy output of the facility. The byproduct, H2, could be used as an energy source in fuel cells or for transportation.
Kim believes that the system design and electrocatalysts can be further improved to make the Na-CO2 cell even more efficient. It will be interesting to see if this invention makes it out of the laboratory to help to mitigate the climate change impact of CO2 while becoming a reliable energy source.
For the fossil fuel industry, I would suggest giving Kim and Jaephil Cho, his colleague at the Georgia Institute, all the money they need to make the Na-CO2 cell commercially viable. It could save their industry and allow the current energy mix to continue to be used along with clean renewable energy sources.