Taking a break from the technology that has allowed us to explore near outer space we turn to inner space, our marine environment. I remember when I was very young and looking at a globe and asking my teacher “why do we call this planet Earth when it is mostly covered by water?” I never got an answer but I guess it has everything to do with our anthroprocentric view as a land animal.
When you look at “Earth” water covers over 70% of the planet’s surface. It is our most dominant feature. More than half of all humans lives within 200 kilometers (120 miles) of a coastline. We are dependent on our water environments which serve as a source of food, an avenue for transportation, a source of recreation, and a place to explore that is both near at hand and yet exotic.
We need water to survive yet only a small percentage of water is drinkable, the rest is filled with salts and minerals washed into it and dissolved from land sources. This undrinkable water, however, feeds our atmosphere, is the source of the water vapour that falls as rain and snow on our continents, and refills our underground aquifers.
Without water Earth would just be “earth,” a dry lifeless place. Yet as precious as it is as a resource we have been reckless with it and have exploited our marine environment to a point where we are creating significant damage. In the next few blog feature articles we will explore this world of Inner Space and what role it will increasingly play in our technical civilization in the 21st century. But first let’s prepare the groundwork or more appropriately the waterworks by looking at some of the Inner Space challenges we currently face.
What is the State of Our Oceans and Seas Today?
I thought I would begin by posing some basic questions about our marine environment to get you thinking about its importance and what we need to do to maintain it in the 21st century and beyond.
1. How much food do we harvest from the oceans today?
Ocean fisheries today provide approximately 16% of the total protein consumed by humans annually. In the Developing World the percentage of protein derived from fish is higher. You would think that most of what we catch gets used for food but in fact, almost 40% is used for other purposes such as fish meal used as a food in aquaculture and as an ingredient in fertlizers. Much of what we catch occurs close to coastlines in water between 10-30 meters (approximately 30-100 feet) in depth. These also happen to be the areas which are most vulnerable to pollutants.
We have overfished much of the planet’s oceans and yet have created fish conservation preserves in less than 1% of the ocean. Almost 90% of the largest game fish stocks have been severely depleted. In Canada we have had collapses in our cod fishery on the Atlantic coast and in our salmon fishery on the Pacific coast.
Aquaculture in the form of fish farms has become a method of commercially breeding fish stocks but there are many challenges to this type of intensive industry.
2. What is the biggest source of pollution in the ocean today?
Is it from land sources, from shipping, from oil rigs, or from some other source? The truth, of course, is pollution comes from all these but land is the main culprit contributing as much as 80% of all that we find in our oceans and marine environments.
The list includes chemicals and oil from industrial sites, fertilizers from farms and urban lawns and gardens, and solid treated and untreated waste, much of it plastic. Plastic garbage constitutes almost 90% of floating debris in the ocean today. Plastic presents a number of problems besides the fact that it floats. It does not easily bio or photo-degrade and when it does it breaks down into smaller pieces that can be ingested by marine animals. Although the plastic eventually fully degrades to its organic roots that process can take centuries. The result is large floating debris fields that now populate parts of the North Pacific and North Atlantic Oceans.
3. What is the average temperature of the ocean today?
Our ocean average temperature has been rising in the latter part of the 20th and here in the 21st century. In 2009 it was 17 Celsius (almost 63 Fahrenheit). Rising temperatures change evaporation rates in the air above the ocean surface and contribute to altered and extreme weather events. Warming oceans also cause water to expand contributing to increased mean sea levels. And warming oceans damage coral reefs causing bleaching and coral death.
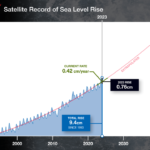
4. Is sea level rising in the world oceans and if so, by how much?
Indeed we have seen a global rise in mean sea levels of between 10 and 20 centimeters (approximately 4 to 8 inches) in the last 100 years. Some scientists refer to this phenomenon as the invisible tsunami putting much of humanity at risk. For places like the Maldive Islands a rise of 20 centimeters is significant, particularly in places where population centres are only 2 meters (76 inches) above mean sea level. Kiribati, another island archipelago is endangered as well with most of its over 112,000 people living on the island of Tarawa , a mere 3 meters above mean sea level. Bangladesh is a nation that appears to be extremely vulnerable to a rise in mean sea levels. A 1.5 meter increase would impact 16% of the country creating 17 million refugees. In addition what rising sea levels mean is storm surges that cause more significant inland flooding, erosion and the despoiling of arable land. And rising sea levels deplete groundwater as saltwater intrudes into inland freshwater aquifers.
5. What is the current impact of increasing CO2 on the world’s oceans?
The ocean today is 25% more acidic than a two centuries ago. During the period of our industrial age human civilization has dumped billions of tons of greenhouse gases into the environment. Half of the CO2 has been absorbed into the ocean creating surface waters containing carbonic acid.
How do we know this? Because we have been measuring the chemistry of ocean water since 1751 using the pH scale (pH stands for “potential Hydrogen,” the ability of molecules to attract hydrogen ions). A neutral pH is 7.0. Liquids that are more alkaline are higher than 7.0 and liquids that are more acidic are lower. Oceans are salty and therefore more alkaline. But today ocean surface waters have an average pH of 8.14. In 1751 the average was 8.25.
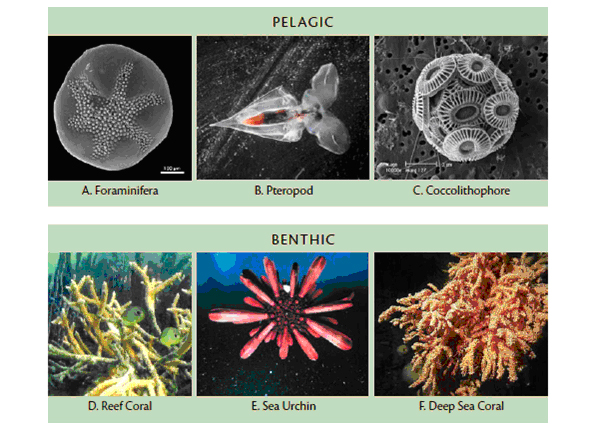
There are implications for creatures that build shells by relying on calcium carbonate available to them in a more alkaline ocean. This includes even the tiniest of ocean creatures – phytoplankton, zooplankton and corals. A decline in plankton impacts the entire food chain in the marine environment including fish, sea birds, invertebrates and marine mammals. The disappearance of coral means the loss of our oceans’ most important marine habitats – the coral reefs.
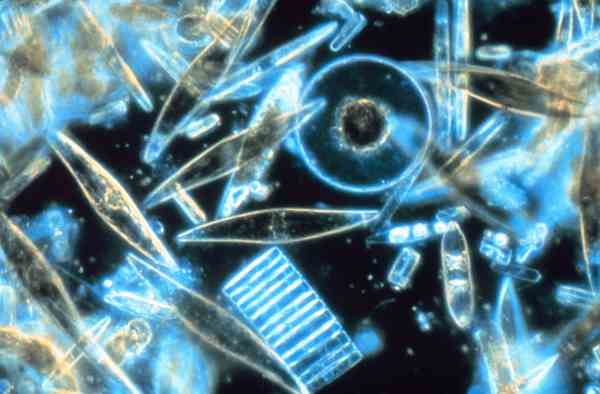
6. How much oxygen does the ocean produce?
Between 50 and 85% of the oxygen we breathe on Earth is produced from the phytoplankton in oceans. Oxygen from phytoplankton has made it possible for air-breathing land animals to evolve and survive. Phytoplankton not only produces the oxygen that these animals breathe it also contributes to the ozone layer in the upper atmosphere protecting us from ultraviolet radiation.
Oxygen in ocean water is essential for marine animal survival. When we contribute to increasing ocean acidity we can impact plankton with devastating consequences. When we see increases in global average temperatures we can see a correlation with the loss of oxygen volume in ocean water leading to ocean dead zones with insufficient oxygen to support life.