Image-guided therapy has revolutionized medicine in the latter part of the 20th century and into these first two decades of the 21st. The operating room, once the exclusive domain of surgeons, is today a very different world. Radiologists, oncologists, cardiologists, nephrologists, lung specialists, gastroenterologists and other medical specialists have invaded the operating room space using sophisticated imaging and interventional technology that replaces surgical procedures.
These disciplines just like surgeons, are more and more using robotics because robots can be far more precise in delivering therapy to a specific location in a patient’s body, and because robots can use technology with no ill effect to the machine, but if undertaken by a physician could unduly expose them to excessive radiation.
In this blog we will look at interventional radiology, cardiology and other medical disciplines to describe where we are using robotics today and where we are headed in the near future.
Today it is hard to tell where the surgical suite ends and radiology begins. Radiology labs like the one illustrated below look as sophisticated as operating rooms.
A Short History of Radiology Before Interventional
Radiology began with a late 19th century discovery by Wilhelm Roentgen, a German scientist. In 1901 he won the first Nobel Prize ever awarded in physics. Roentgen’s first application of the technology involved x-raying his wife’s hand including the wedding ring she was wearing. X-ray technology became a standard in the first half of the 20th century as a diagnostic tool for everything from skeletal injuries to welded metal plate seams.
In the 1950s contrast agents made it possible to use X-ray technology to see soft tissue and body organs. Development of X-ray movies made it possible to view internal organs at work expanding the capability of this technology as a diagnostic tool.
The 1960s saw the development of sonar imaging using ultrasound, sound wave frequencies of 20,000 or more vibrations per second. The marriage of ultrasound and computer software further perfected this new imaging technology leading to today’s 3D and 4D ultrasounds used in looking at heart, kidney, uteri and abdominal organ studies. Today expectant mothers and fathers throughout much of the world can get a first look at their future child through fetal ultrasounds. Some even post these images on Facebook.
The 1970s saw the emergence of computed tomography or CT, the digital mapping of the human body producing detailed images of anatomy and physiology.
In the 1980s magnetic resonance imaging or MRI was added to the arsenal of diagnostic tools, creating images in fine slices that could be assembled to provide detail never seen before.
Interventional Radiology
Moving X-rays made it possible for radiologists to become more than diagnosticians. I first encountered the power of X-ray in this format as a new father when my daughter underwent a cardiac catheterization study involving the insertion of a catheter into a vein in her leg that was threaded into her major blood vessels and heart. A cardiologist injected contrast dye into the catheter to measure my daughter’s pulmonary arteries, heart chambers, lungs and study her blood flow patterns. The results confirmed a diagnosis of complex heart and lung disease, first imaged through ultrasound, and now studied in greater detail using X-rays. Although purely diagnostic in nature these initial catheterization my daughter experienced were forerunners of what is today a significant medical breakthrough, the implanting of medical devices to repair the body using catheters.
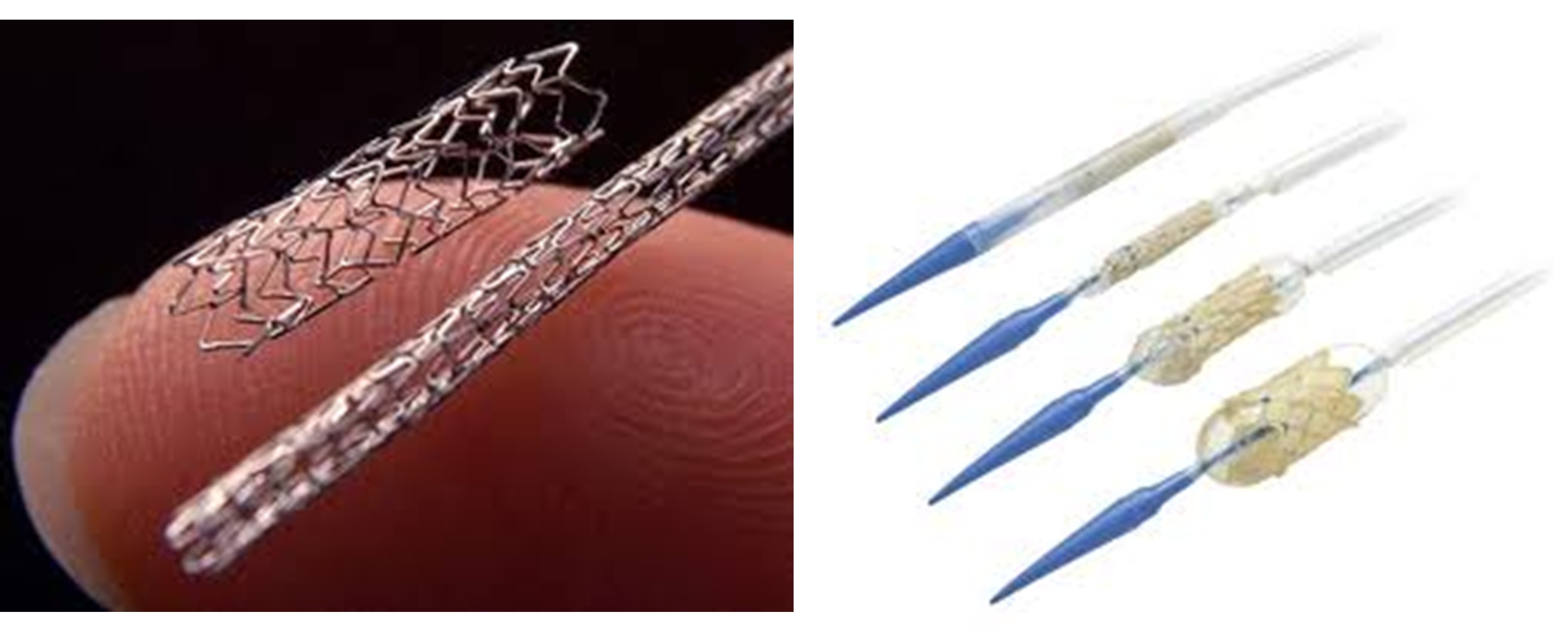
Today radiologists, cardiologists and other specialists routinely do procedures called angioplasty, using catheters with balloon tips that are inflated to open blocked arteries, or to insert stents and heart valves into patients (see pictures below).
Catheters give radiologists other means to deliver treatment. A catheter can be outfitted with a heatable tip containing a platinum electrode with a temperature sensor. Using radio-frequency (microwave) energy, the catheter can literally nuke tissue in the body to kill it. Called radio-frequency ablation or RFA, catheterizations of this type cure arrhythmias (abnormal heart rhythms), and reduce cancerous tumors in the liver and esophagus. A catheter containing an optical fiber can use lasers to treat cancers. Called laser-induced interstitial thermotherapy or LITT, the laser can deliver heat to destroy or shrink a tumor. Another laser treatment called photodynamic therapy, or PDT, activates photosensitized agent chemicals that specifically target cancer cells.
Catheters can deliver chemicals and drugs to a body site to attack a tumor or destroy abnormal tissue. A procedure developed in 1994, called alcohol septal ablation (ASA) is commonly used today to treat a deadly heart condition called hypertrophic cardiomyopathy, where abnormal muscle thickening reduces the heart’s ability to pump leading eventually to blockage of the aorta, the main artery in the body. In the case of the latter the alcohol is injected directly at the point of the muscular blockage site to kill the excessive tissue restoring normal blood outflow.
Catheters can deliver freezing fluids to perform cryoablation or cryotherapy, used to treat prostate, liver and some forms of bone cancer.
Enter the Robots
As interventional treatments have become more the norm than the exception in dealing with many complex health and disease problems, the need for precision and accuracy has led to the development of robotics devices. Some of these devices provide real-time magnified imaging in 3D. Others are used in conjunction with surgical applications to assist in minimally invasive procedures. Most are designed to reduce exposure of both patient and physicians to X-ray irradiation with the latter able to operate these devices remotely.
Siemens has been a pioneer in robotics in manufacturing and other industries and is now applying this expertise to the medical field by building a number of systems and devices. One of these is Treago, an image-guided robotic treatment table designed to position patients for radiation therapy treatments. Other Siemens devices include Artis Zeego, an radiology system capable of rotating 360 degrees in 6 seconds producing detailed images of entire tumors including blood vessel feeds. Because Artis Zeego is so fast it minimizes patient exposure to X-rays and radioactive contrast.
The CyberKnife, a product of Accuray, a California-based company, features a robotic arm capable of moving around a patient to deliver precise radiation to tumors with sub-millimetre accuracy. The system can sense movement in the patient such as the rise and fall of the chest during breathing and adjust the radiation beam to compensate. How does the CyberKnife know where the tumor is located? The technology communicates with CT scanners and uploads data from these sources to create an accurate location map for the targeted tumor and for healthy surrounding tissue. The medical team creates a treatment plan for the CyberKnife along with desired radiation dosage. CyberKnife’s precision allows for larger doses of radiation focused on the cancer, not healthy cells. Where typically a patient would undergo 20-30 visits using conventional radiation therapy, a CyberKnife treatment plan requires from one to 5 sessions. Because of this precision CyberKnife can treat complex, dispersed and inoperable tumors in the prostate, lung, brain, spine, liver, pancreas and kidney.
As the 21st century continues to unfold we will see more robotic-assisted technology in radiology designed to be less invasive and more precise in treating cancers and other diseases.
Cath Lab Robotics
The same can be said about the use of robots in cath labs where computer-guided catheters combined with navigation systems are allowing cardiologists to do procedures that were once far more invasive and done exclusively by surgeons.
The first use of a robotic system in cardiology dates to 1997. The robot was named Aesop (see our previous blog). Today, catheter-based therapies increasingly rely on robot-assist devices for both diagnostic and interventional procedures. These labs include catheter manipulation by robots directed by computer-aided imaging, and therapeutic robotic devices.
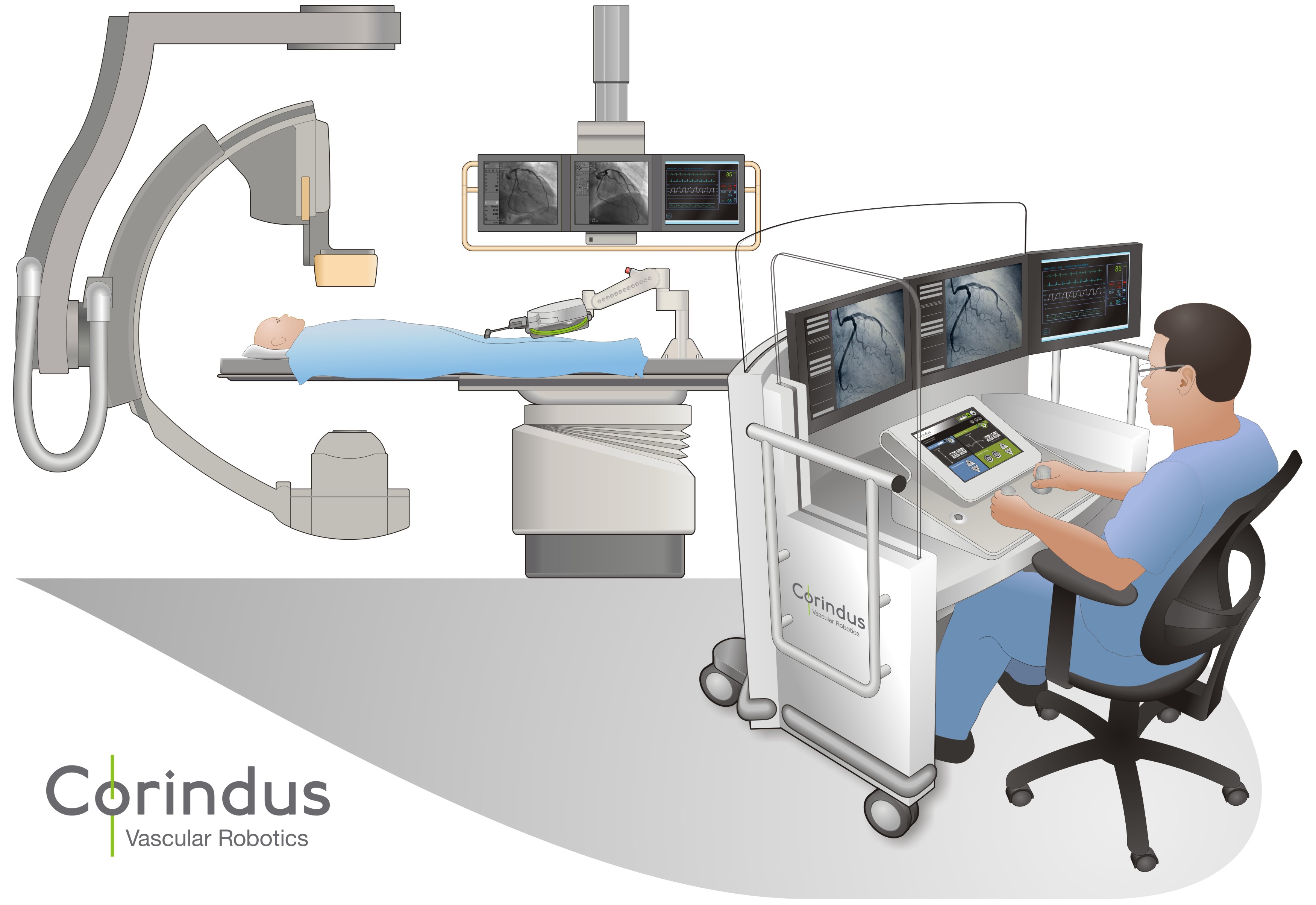
Corindus Vascular Robotics, in Massachusetts, is the developer of the CorPath 200, a robotic system for doing percutaneous coronary intervention procedures, called PCIs. Remember when I mentioned my daughter in this article. Less than 3 years ago she had a PCI done to implant a pulmonary valve and stent. But in her case it was done without robotic assistance and involved a full cardiac team of doctors and nurses. CorPath 200 provides an articulated robotic-arm combined with a remote cockpit containing a workstation with multiple screens and joystick controllers. The cardiologist, from the comfort of the cockpit, does a procedure using the robot arm to accurately place stents and other implanted devices using balloon catheters.
Even more interesting are robotic devices that can get inserted into the chest cavity and placed on the surface of a beating heart. One of these is HeartLander, a miniature mobile robot designed to facilitate minimally invasive therapy. Designed to adhere to the outer surface of the heart (the epicardium), this devices moves like an inchworm to position itself on the heart muscle for administering a variety of therapies.
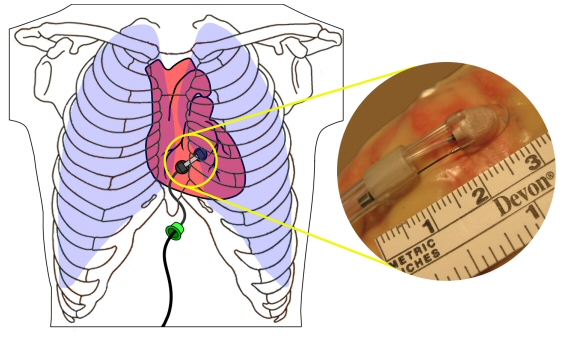
It navigates autonomously using suction to adhere to the surface and an internal drive wire to create push and pull. The cardiologist can control HeartLander using a joystick and view its progress through a graphical computer interface. The current device is 8.5 mm in diameter with plans to shrink it further to 3 mm.
HeartLander in trials has been used for ablation treatment of arrhythmias, to place leads on the heart muscle for pacing and for delivering medication and chemicals to targeted areas of the heart muscle.
Currently still in the laboratory and being used in animal studies, HeartLander represents the next stage in the evolution of robotic systems for use in biomedicine.