April 10, 2014 – Using agricultural land to grow an industrial crop to convert to fuel seems like a make work project for farmers. Yet an entire subsidized industry has grown up in Brazil, the United States, Canada and other countries doing just that. In Brazil they turn sugar cane into ethanol (which I don’t mind too much since sugar in my book is more a food additive than a food). In North America, however, it is corn that we use to produce biofuel. I know it’s corn that we wouldn’t grow for human or animal consumption but it is tying up acreage that could be growing food. And it would seem to me that agricultural land, even that considered marginal, could be put to better use.
That’s why what Stanford researchers have discovered could prove to be a game breaker, a far less resource intensive way to produce ethanol and other biofuels. What are they doing? Synthesizing ethanol using copper catalyst, CO2 and water by first converting the CO2 to CO (carbon monoxide) and by applying electricity yielding ethanol. What makes this potential technology (it still is in the laboratory at present) so exciting is its scalability. All of us could start producing ethanol simply by using a power source at home like solar panels on our roof, harvesting CO2 from the air, adding water and producing ethanol. That ethanol could serve to power our homes when the Sun is not shining. Or we can use it as fuel for our transportation requirements. On a larger scale existing coal-fired power plants would be able to capture the CO2 they exhaust into the air, use the copper catalyst, add water and electricity and create ethanol in significant volume for resale.
How does it work? The key is in the copper catalyst. Copper uniquely can reduce CO in the presence of water converting it to ethanol but in the past it has proved to be highly inefficient. The Stanford team discovered that by altering the copper metal, heating it in air to form a layer of copper oxide, they could then treat the oxidized surface chemically to create a network of copper metal nanocrystals (see the image below). They found when they applied electricity they saw a ten-fold increase in the copper’s efficiency in converting CO and water to ethanol. Now they are experimenting to produce other biofuels such as propanol which has an even higher energy density than ethanol.
Can we humans have our cake and eat it too because of what these Stanford scientists have discovered? Think about it. If we can harvest CO2 from the air, efficiently convert it to CO and then manufacture chemical fuels we will have created the 21st century’s answer to controlling greenhouse gas emissions. You can read the published paper on this research in the latest issues of the journal Nature.

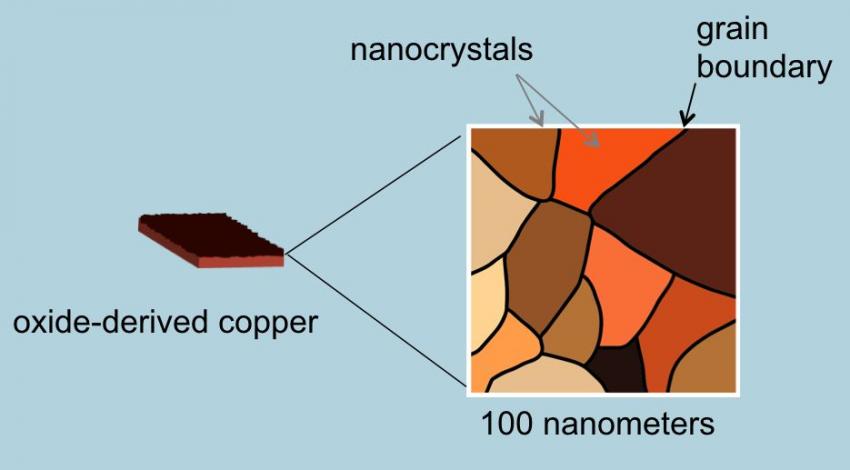

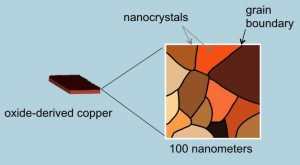


















[…] Stanford researchers create a copper catalyst that can harvest biofuels from CO2 in the air. Can this help us with global warming? […]
The idea of obtaining large amounts of 300-400 ppm CO2 from air is probably not economically feasible. This problem has already been well studied decades ago. Nuclear submarines contain air for their 100-man crews to breathe, and each crewman’s respiration constantly adds CO2 to the air. Hence it’s vital that some system remove the CO2. WWII diesel-powered subs rarely stayed submerged for more than a few hours, and when they did the air soon became foul and as a general practice the crews spread bara lime on the bunks. The bara lime worked well enough, and it was cheaper than more reactive chemicals.
A similar chemical system was used in the Apollo lunar missions, which employed lithium hydroxide in canisters as the CO2 scrubbing agent. Nuclear subs, the space shuttle, and international space station, with much more power available, use regenerative systems. But the fundamental concept remains the same: some chemical reaction lightly binds the CO2. Heating the resulting compound releases the CO2, which can then be vented. A lot more energy is consumed in the processes than would ever be released by converting the recovered CO2 into CO to use in a liquid fuel synthesis process.
From Wiki: “The dominant application for CO2 scrubbing is for removal of CO2 from the exhaust of coal- and gas-fired power plants. Virtually the only technology being seriously evaluated involves the use of various amines, e.g. monoethanolamine. Cold solutions of these organic compounds bind CO2, but the binding is reversed at higher temperatures:
CO2 + 2HOCH2CH2NH2 HOCH2CH2NH3+HOCH2CH2NH(CO2-)
As of 2009, this technology has only been lightly implemented because of capital costs of installing the facility and the operating costs of utilizing it.” The basic chemistry hasn’t changed, and it probably never will.
See: http://www.nytimes.com/2013/01/06/business/pilot-plant-in-the-works-for-carbon-dioxide-cleansing.html?_r=0
and: http://en.wikipedia.org/wiki/Carbon_dioxide_scrubber
Carbon Engineering’s program sounds like another uneconomic greenhead technical assault on public funds. But even Carbon Engineering is not seriously contemplating extracting CO2 from the air. If they are going to harvest any CO2 from a mixture of gases, it will be from the exhaust of coal-fired power plants (about 150,000 ppm CO2). Only if public funds subsidize it would the harvested CO2 project be profitable to Carbon Engineering. Black Light Power and Rossi’s E-cat look like bad bets, Carbon Engineering’s scheme, while at least technically possible, looks even worse.
Hi Al, Thank you for you input. The Stanford technology is using copper with water and electricity to take CO and convert it to ethanol. The source of the CO is CO2 which obviously has to be stripped of one oxygen atom by some industrial or chemical process. I would assume that there are inexpensive ways to do this. I know researchers at University of Delaware invented a process using electricity, water and bismuth. You can read about that discovery at: http://www.udel.edu/udaily/2013/jun/solar-synthetic-fuel-062013.html. So it would seem that what Stanford is developing may be an interesting solution. As for the carbon capture, extracting CO2 out of the air I thank you for your input.
((So it would seem that what Stanford is developing may be an interesting solution. As for the carbon capture, extracting CO2 out of the air I thank you for your input.))
Well I just figured there is little point of trying to assess the merits of step two process (conversion of CO2 into CO), if step one, obtaining the CO2, isn’t feasible. So maybe step two could make sense if the CO2 is obtained by some means other than extracting it from a mixture of gases. The difficulties of extracting CO2 from a mixture of gases seem to make that approach uneconomical; hence the conversion to fuel would also be uneconomical. Maybe a fair question would be whether Carbon Engineering could make a free market profit with its copper catalyst process even if it were given infinite amounts of pure CO2 absolutely free?
I see there are production facilities in Germany converting CO2 and water to methane for home heating. So I am curious, what will end up the less expensive proposition. And does this represent the possibility of methane powered ships? Maybe for the ships carrying beef and lamb on the hoof, fresh to market.
[…] Converting carbon monoxide to ethanol could prove revolutionary. […]