October 11, 2016 – In March of this year 21-year old Kris Boesen was in a car accident and suffered severe trauma to his cervical spine. His diagnosis, permanent paralysis from the neck down. He would be a quadriplegic for the rest of his life. But doctors at the University of Southern California Neurorestoration Center attempted an experimental treatment using stem and other cells which were introduced into the damaged cervical spine.

Two weeks post surgery Boesen (seen in the picture above) showed some signs that the procedure was working. And after 90 days he could feed himself, write his name, use a cellphone, hug his friends and more.
Explains Dr. Charles Liu, Director of the Neurorestoration Center, “Typically, spinal cord injury patients undergo surgery that stabilizes the spine but generally does very little to restore motor or sensory function….with this study, we are testing a procedure that may improve neurological function, which could mean the difference between being permanently paralyzed and being able to use one’s arms and hands. Restoring that level of function could significantly improve the daily lives of patients with severe spinal injuries.”
Liu reports that 90 days post surgery Boesen is demonstrating motor function that should it continue to improve will allow much greater levels of functional and physical independence than found in current standard treatment.
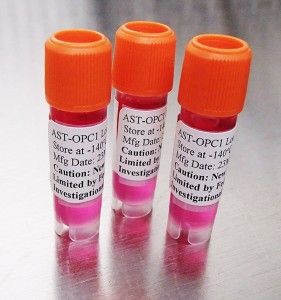
There are six sites in the United States currently enrolling candidates for clinical trial of this new procedure which involves escalating doses of AST-OPC1 cells (see below) developed by Asterias Biotherapeutics. The cells are made from embyronic stem cells converted to oligodendrocyte progenitor cells, cells found in the brain and spinal cord. AST-OPC1 cells in laboratory studies has been shown to stimulate myelination of damaged axons as well as development of new blood vessels.
Clinical trial sites include Indiana University in Indianopolis, Medical College of Wisconsin in Milwaukee, Rush university in Chicago, Shepherd Center in Atlanta, Stanford University and Keck Medicine at University of Southern California.