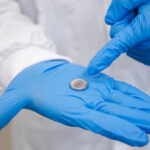
A Toronto-based, Canadian scientist is in the news today. Dr. Molly Shoichet, who holds the Canada Research Chair in Tissue Engineering, is in charge of a laboratory at the University of Toronto focused on engineering new materials and treatments in the fields of regenerative medicine, tissue engineering, and drug delivery. Along with her team she has designed a novel injectable hydrogel for postoperative pain management that eliminates the need for opioids such as morphine, morphine derivatives, Oxycontin and Oxycodone. Dr. Shoichet’s research has led to a lab spinoff called AmacaThera Inc., a biotechnology company focused on commercializing the discoveries.
In a Globe and Mail Report on Business article written by Sean Silcoff, Dr. Shoichet describes the drug delivery invention as the equivalent of a FedEx with the customer our body’s cells. Her explanation of the analogy goes as follows: “FedEx provides the packaging and figures out how to get what’s inside where and when it needs to be there.” That’s exactly what Dr. Shoichet’s hydrogel does, packaging that releases the contents inside it once delivered to the patient. In the case of postoperative pain management, the hydrogel releases bupivacaine gradually over 72 hours, a period of time sufficient to get most patients over the hump to a point where they don’t need opioids. The gel itself is a combination of hyaluronic acid, a chemical found naturally in our bodies and in ointments and cosmetics, combined with methylcellulose.
Hance Clarke, an anesthetist at the University Health Network in Toronto sees the value in a therapeutic pain management option that lasts 72 hours after surgery. In the Silcoff article, he states that AmacaThera’s biotechnology would extend the best anesthetic pain management solutions available today by an additional 48 hours. He concludes that this would have real clinical value.
Why was Dr. Shoichet’s work featured on the business pages of Canada’s national newspaper? Because her company, AmacaThera, has just raised $10.3 million from Canadian, U.S., and European investors. This follows an initial seed funding of $1.8 million USD in 2018.
Back in October 2020, Health Canada gave AmacaThera permission to begin a Phase 1 clinical trial of its pain management delivery system. At the time Dr. Shoichet stated, “Amaca gel has shown broad utility with an array of small-molecule drugs, biologics, and cells for many different indications.”
That’s the interesting spin in this story. Using a gel as an envelope for drug and other forms of therapeutics has an interesting parallel with mRNA vaccine technology, particularly the one produced by Pfizer-BioNTech. It uses a lipid nanoparticle as its equivalent to the analogous FedEx envelope of Dr. Shoichet’s explanation. The content is Messenger RNA (mRNA) which then begins to build a patient’s immunity to fight COVID-19. Interestingly, this lipid nanoparticle also comes from Canadian research but this time at the University of British Columbia. The technology also has been commercialized by a university spinoff company, Acuitas Therapeutics.
Without the envelope, in both AmacaThera and Acuitas, the critical medications contained therein, would not be able to be delivered to patients, whether for pain management or a vaccine to fight the COVID-19 pandemic.